What is Glucosyl stevia?
Glucosyl Stevia is a stevia-based flavors and flavor modifiers for use in food and beverage products. It is also called Glucosyl Steviosides, Enzymatically Modified Stevia Glucosyl Stevia or Enzymatically Modified Stevia.
Glucosyl Stevia is made by the selective introduction of Glucosyl into Steviol glycosides molecule with the biological enzyme technology, which increases the number of glucosides. When compare with Steviol glycosides, it has many advantages, such as it can reduce Stevia’s natural bitter tastes and increased solubility.
Definition in JP (Japan Phamaceutical):Enzymatically Modified Stevia is obtained by addition of glucose to stevia extracts using α-glucosyltransferase. Its components are α-glucosylstevioside, etc.
Definition in China: Glucosyl Steviol Glycosides is obtained by Stevia Rebaudiana Bertoni Leaf as raw material, Steviol glycosides extracted from stevia leaves enzymatically glycosylated, and then concentrated by evaporation, spray-dried glucose-based stevia glycosides.
Glucosyl Stevia Characteristics
It greatly improves Steviol glycosides’ quality and taste. Glucosyl Stevia is white or light yellow powder or granular, it tastes cool and sweet, the sweetness is 100-150 times higher than that of sugar. Glucosyl Stevia is manufactured from Steviol glycosides, and with the bio-enzyme fermentation technology, the molecule of Steviol glycosides is attached with glucosyl groups, which forms a new Steviol glycosides. This kind of stevia overcomes the different purity issue and bitterness-aftertaste of common Steviol glycosides, which has a better sweetness.
Usually, lactose and maltodextrin are used as the dispersant to reduce the sweetness to 0.5-1 times that of sugar.
Glucosyl Stevia Application and Use
It is stable under usual food processing conditions. Its water dispersion is increased with enzyme technology. As this result, its solubility is higher, making it quite suitable for various beverages and wines. It’s mainly used in low calorie food, sodas, juices, cold drinks, desserts, pickles and aquatic products.
Glucosyl Stevia Manufacturing process
Stevioside glucosylation refers to a process in which the glucose residue provided by a glycosyl donor is transferred to a natural steviol glycoside under the action of one or several enzymes, that is, an enzyme-catalyzed transglycoside reaction, commonly known as an enzyme.
There are the following key points in the manufacturing process: raw materials, biological enzymes, glycosyl donor, reaction conditions
Raw natural stevia glycosides
Raw materials such as Rebaudioside –A series: 90% – 98% Rebaudioside-A or 40% – 80% Rebaudioside-A; Stevioside 90%- 95%, Steviol Glycosides: 80%-98% Steviol Glycosides and etc. I think you can believe different raw materials react to Glycosyl can get the different taste of Glucosyl Steviol Glycosides although the difference may not much.
Biological enzymes
The choice of enzyme cyclodextrin glucosyltransferase, α-glucosyltransferase, β-amylase, etc., I think you can believe maybe different Biological enzymes can get different Glucosyl Steviol Glycosides.
Glycosyl donor
Starch, maltodextrin, or other dextrins, etc., different glycosyl donor can affect the quality?
Reaction conditions
temperature, pH, reaction time, etc., different reaction conditions have an impact on product quality?
Why Glucosyl Stevia appears in the market
As an alternative to sucrose, stevia provides a natural and sweet taste with zero-calorie and healthy. In recent years, steviol glycosides has been widely accepted in the market, especially in USA. In order to provide sweet taste and reduce calorie intake,
Stevia has been used in many food and beverage products and labelled as “Clean Label”in the US market for the past two years.
Natural Steviol glycosides with non-toxic side effects and food safety. Regular consumption can prevent high blood pressure, diabetes, obesity, heart disease, dental caries and etc., It is recognized as the third generation of sugar alternative. However, it has the unpleasant taste feeling due to its own structural steviol, which has no sweet taste but bitter taste after eating, that seriously hinders its application in the fields of food and beverage.
Therefore, people hope that the taste of Steviol glycosides can be improved through a certain research and treatment.
As the steviol structure in natural steviol glycosides structure has a certain bitter taste. And due to the number of glycosides linked to the molecular structure of steviol and the location of different positions, some steviol glycosides products will have a bitter astringency, which inevitably affects the end products taste. Glucosyl Stevia, it is steviol glycosides linking with glycosyl in the designated parts of the stevia glycosides structure through the enzyme, to improve the natural stevia glycosides taste.
The technology began in Japan in the 1880s. Especially in the past 10 years, many companies carried out the GRAS declaration and related patent applications on enzymatic stevia in different countries or regions.
China Glucosyl stevia market
China is a major exporter of steviol glycosides. In 2016, its steviol glycosides annual export volume of reached 240 million U.S. dollars, with 5137 tons of various types of steviol glycosides products. In the first three quarters of 2017, exports reached 170 million U.S. dollars, and all kinds of steviol glycosides products reached 4221 tons. The export volume increased by 19% over the same period of last year. The amount of exports to the United States in 2016 accounted for about 26.4% of the total volume of exports reached 8.94 million US dollars in the first three quarters of 2017 accounted for 30.4% of the total exports reached 7.76 million US dollars.
From the above China export data, we can see the popularity of steviol glycosides in US market. As a result of all steviol glycosides use the same HS code, so the export data of glucosyl stevia cannot clearly be seen so we don’t how many quantity of glucosyl stevia have been export to US market in recent years.
To our surprise, a USA company sued several companies who selling glucosyl stevia products in the United States claimed against their patent disputes.
https://www.usitc.gov/press_room/news_release/2017/er1120ll869.htm
From the patent disputes, we should think: how many quantity of glucosyl stevia are used in the US market per year and how many quantity of glucosyl stevia in steviol glycosides are exported to US from China?
Global quality standard on Glucosyl Stevia
1. China standard: according to GB2760-2014 quality standard
| Test Method | Specification | |
| Glucosyl Stevia(GSG),w/% ≥ | 75.0 | 附录A中A.3 |
| Rebaudioside A +
Stevioside ,w/% ≤ |
6.0 | |
| Rebaudioside A,w/% ≤ | 4.0 | |
| Stevioside,w/% ≤ | 4.0 | |
| Maltodextrin,w/% ≤ | 20.0 | |
| Rotation | +65°~ +75° | GB/T 14454.5 |
| Relative Density | 0.2~0.6 | GB/T 11540 |
| pH | 4.5~7.0 | GB/T 9724 |
2. Japan Standard
| α-Glucosyl Stevia, % | ≥ 75.0 |
| Steviol glycosides, % | ≤ 15.0 |
| α-Glucosyl Stevia+
Steviol glycosides, % |
≥ 85.0 |
3. US GRAS Notice (GRN) No. 662 on Glucosylated Stevia Leaf Extract
Raw materials is Rebaudioside A 95
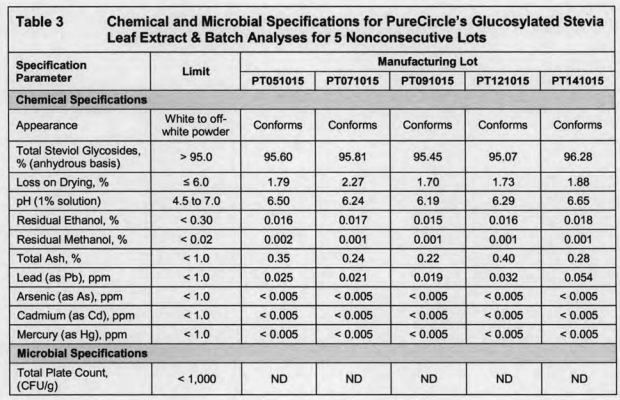
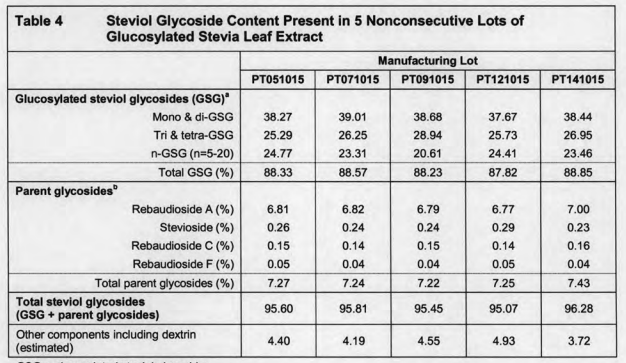
We can see from this documents that if write down the data as China standard would be as follows:
| Test Item | Lot
PT051015 |
Lot
PT071015 |
Lot
PT091015 |
Lot
PT121015 |
Lot
PT141015 |
| Glucosyl Stevia w/% | 88.33 | 88.57 | 88.23 | 87.82 | 88.85 |
| Rebaudioside A +
Stevioside w/% |
7.07 | 7.06 | 7.23 | 7.26 | 7.23 |
| Rebaudioside A w/% | 6.81 | 6.82 | 6.79 | 6.77 | 7.00 |
| Stevioside w/% | 0.26 | 0.24 | 0.24 | 0.29 | 0.23 |
Source from: https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm519307.pdf
When compared with China standard, you’ll find that here Rebaudioside A + Stevioside w/% exceed 6% and Rebaudioside A,w/% exceed 4%. That is to say, if the GRAS Glucosyl Stevia using Rebaudioside A 95 raw materials can not meet China quality standard.
China approved Glucosyl Stevia since in 2016. Different countries have different quality standard. Let’s see what will happen in new future.